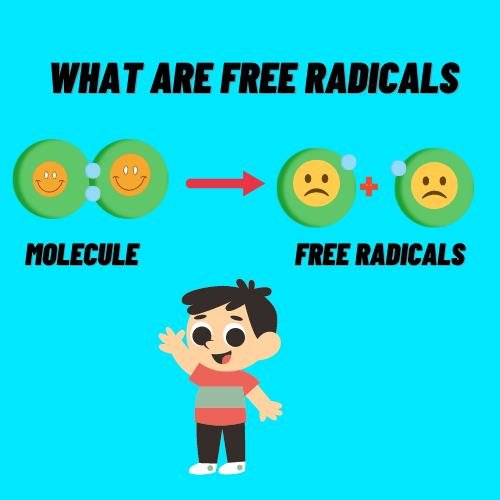
What is radical in chemistry
- Free Radical Is Defined As Any Atom Or Molecule Possessing Unpaired Electrons.
- A Free Radical As Any Molecular Species Capable Of Independent Existence With An Unpaired Electron In Atomic Orbital.
- So, these atoms, called free radicals, scavenge the body to seek out other electrons so they can become a pair.
* The free radicals cause the damage to the bio-molecular structure.
* Attacks important macromolecules leading to cell damage and homeostatic disruption.
* Targets of free radicals include all kinds of molecules in the body.
* Major targets & Adversely altered are carbohydrates, lipids, proteins, and DNA.
* The increased amount of free radicals causes various diseases.
* like cancer, diabetes, cardiovascular diseases, autoimmune disorders, neurodegenerative diseases, aging etc.
Note:
* Moderate Levels of ‘Free Radicals’ Found Beneficial to Healing Wounds.
* “Free radicals” generated by the cell’s mitochondria.
* The energy producing structures in the cell are actually beneficial to healing wounds.
FEATURES OF FREE RADICALS
* The presence of an unpaired electron results in certain common properties that are shared by most radicals.
*Free radicals can either donate an electron to or accept an electron from other molecules.
* Free radicals behave as oxidants or reductant .
★ Free Radicals,
- Are Electrically Neutral Molecules,
- Are Highly Unstable,
- Are Electron Deficit Atoms,
- Are Found To Be Excited/Very Reactive,
- Are Paramagnetic In Nature,
- Are To Generate An Other Free Radical By Extracting An Electron Of Stable Molecule,
- Are Combined Together To Form Neutral Molecule.
TYPES OF FREE RADICALS
- Reactive oxygen species/intermediates (ROS/ROI)
- Reactive nitrogen species/intermediates (RNS/RNI)
★ROS is composed of
- Superoxide Anion (02),
- Hydroxyl (OH.),
- Hydroperoxyl (OOH),
- Peroxyl (ROO.)
- Alkoxyl (RO.)
-Radicals Non Free Radicals Are
-Hydrogen Peroxide ( H₂O₂ ), Hypochlorous Acid ( HoCl ),
-Ozone (03) Singlet Oxygen (10₂).
-Oxygen Species (Ros).
★ RNS are mainly
– Nitric Oxide (NO)
– Peroxynitrite (ONOO)
– Nitrogen Dioxide ( No₂).
FREE RADICALS FORMATION
- Homolytic cleavage of a covalent bond, in which a normal molecule fragments in two, each fragment retaining on of the paired electrons.
- Homolytic cleavage occurs less commonly in biological systems, as it requires high-energy input from ultra-violet light, heat or ionising radiation.
What are Free Radicals?
★Free radicals are like robbers which are deficient in energy
★Free radicals attack and snatch an electron from other cells to satisfy themselves… thus damaging cells membrane.
★The chemicals cortisone and catecholamines created by mental stress can create free radicals.
★Our bodies – Free radical molecules are a natural byproduct of cell metabolism.
★Alcohol – Consumption of alcohol of any kind of any amount produces free radicals in the body.
★Polyunsaturated fat like that found in vegetables oils is easily oxidized in the body and can create
★free radicals. Substitute polyunsaturated fats with monounsaturated fat.
FREE RADICALS SOURCE
■The Source Of Free Radicals Formation in
1. Internal Source Of Free Radicals Generation It Is In The Cells Metaboc Processes Of Human Body
As a source are Enzymatic reactions; those are the respiratory chain, in phagocytosis, in prostaglandin synthesis, and in the cytochrome P-450 system.
Mitochondria, Xanthine oxidase, Peroxisomes, Inflammation, Arachidonate pathways, Exercise, Ischemia/reperfusion injury are the internal sources.
2. From External Sources Of Free Radicals Generation
It is by Non-enzymatic Reactions Of Oxygen With Organic Compounds As Well As Those Initiated By lonizing Reactions.
External sources are like Cigarette smoke, Environmental pollutants, Radiation, Certain drugs, pesticides, Industrial solvents, Ozone, X-rays etc
-
Xanthine oxidase is a form of xanthine oxidoreductase, A type of enzyme that generates reactive oxygen species.
- These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid.
- These enzymes play an important role in the catabolism of purines in some species, including humans.
Ischemia-reperfusion
injury (IRI) or reoxygenation injury is the tissue damage caused when blood supply returns to tissue (re- + perfusion) after a period of ischemia or lack of oxygen (anoxia or hypoxia).
FREE RADICALS PRODUCTION IN CELLS
■The production of free radicals in cells can happen both accidentally or deliberately
■ The Accidental generation of free radicals would be the leakage of superoxide, hydrogen
■ peroxide & other ROS at the interface of the bacterium & the activated phagocyte.
■ As a activated phagocyte ingests foreign particle, its oxygen consumption increases and the phenomena is called a RESPIRATORY BURST,
■This process of RESPIRATORY BURST produces reactive oxygen-containing molecules that are anti-microbial.
■ The oxygen containing molecules are toxic to both the invader and the cell itself, so they are kept in compartments inside the cell.
■ This method of killing invading microbes by using the reactive oxygen-containing molecules is referred to as oxygen-dependent intracellular killing, of which there are two types.
What is frequently asked questions?
How do you get free radicals?
Ans:- There are a few ways to get free radicals. One way is to consume foods that are high in antioxidants. Antioxidants help to neutralize free radicals in the body. Another way to get free radicals is to exercise. Exercise helps to increase the production of free radicals in the body.
What Is Radical In Chemistry? Give Example.
- 1. What is a radical in chemistry?
- 2. What are some examples of radicals?
- 3. How do radicals differ from other atoms or molecules?
- 4. What are the consequences of having radicals in chemistry?
- 5. How can radicals be prevented or removed?