Introduction
The laboratory method of preparation of hydrochloric acid involves mixing a concentrated solution of sodium chloride with sulfuric acid. This reaction produces hydrogen chloride gas, which is then dissolved in water to create hydrochloric acid.
The necessary safety precautions for this reaction should always be followed, such as wearing safety glasses and gloves, as the reaction produces corrosive gases. Additionally, the reaction should be conducted in a well-ventilated area as the gases can be harmful if inhaled.
Hydrochloric acid
Hydrochloric acid (HCl) is a colourless, corrosive, highly acidic solution of hydrogen chloride in water. It is commonly used in chemical production, industrial cleaning, and pickling applications. It can also be used to treat drinking water and wastewater.
Thanks for your question!
How do I prepare hydrochloric acid in laboratory? Or Describe Briefly Them Ethod Em Ployed to Dissolve Hydrogen Chloride Gas in Water as It is Prepared. – Chemistry.
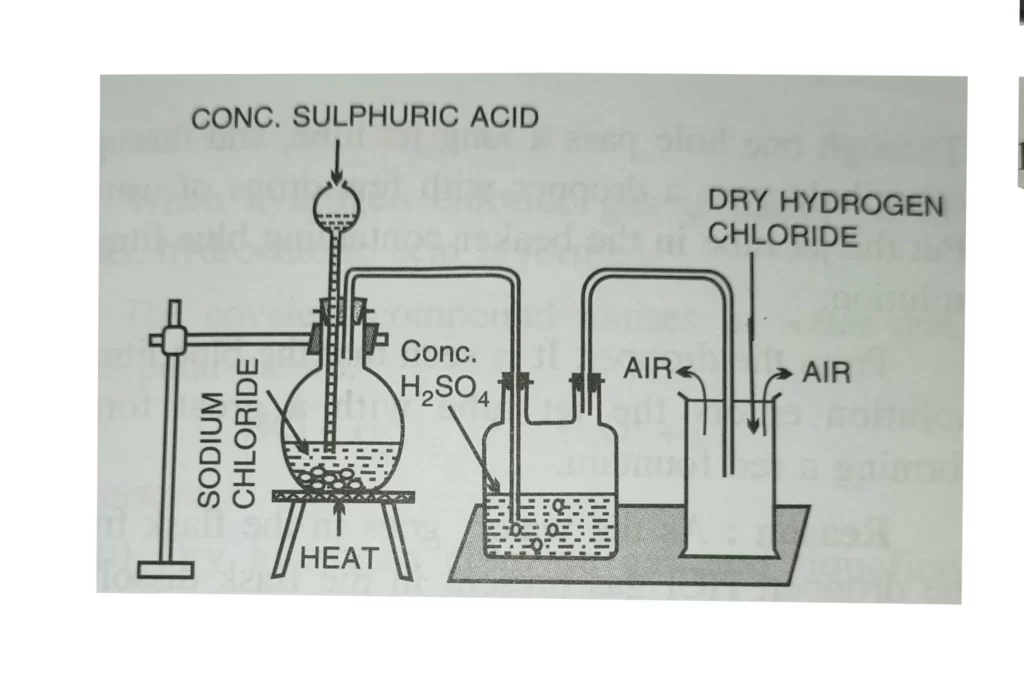
Preparing hydrochloric acid in laboratory is a relatively simple process. First, hydrogen chloride gas must be obtained. This gas can be generated in a fume hood by combining concentrated sulfuric acid and common table salt. Once the gas has been generated, it must be dissolved in water. This is most easily done by bubbling the HCl gas through a solution of water and concentrated sulfuric acid. This allows the HCl gas to dissolve in the water and form hydrochloric acid. It is important to be cautious and wear protective gear as HCl is corrosive and can be dangerous.
Fabrication of Hydrogen Chloride
Hi there! Fabrication of hydrogen chloride requires the use of hydrochloric acid and hydrogen gas. The hydrogen gas should be bubbled through the acid and collected. To ensure a safe fabrication process, it is important to wear the appropriate safety equipment such as eye protection, gloves, and a face mask. Make sure to take all necessary safety precautions when working with hazardous chemicals. Thanks for your question!
Some Important Reactions of HCl
HCl is a strong acid that has many important reactions. It is used as a source of the hydronium ion (H₃O+) in water-based solutions, and it can be used to neutralize basic compounds such as sodium hydroxide (NaOH). It can also be used to react with a variety of other compounds, such as metal oxides, to form corresponding chlorides. In addition, HCl can be used to catalyze reactions, such as the Fischer – Tropsch process and the Aldol reaction. Finally, HCl can be used to protonate certain organic molecules, such as alcohols, to form alkyl chlorides.
Solved Example for You Hcl
Hi there,
I understand that you are looking for a solved example for Hcl. Here is an example that may help you out:
HCl is a strong acid and is one of the most commonly used acids in a laboratory. To illustrate an example of its application, consider a titration experiment. A titration is a lab technique used to determine the concentration of a solution. In this example, we will use 0.1 M HCl as the titrant and a 0.05M NaOH solution as the analyte.
To begin a titration, you will first need to determine the volume of HCl needed to neutralize the solution. To compute this volume, we can use the equation V = [ C1V1 = C2V2 ], where V is the volume of the titrant, C1 is the concentration of the titrant, and C2 is the concentration of the analyte. In our case, V = [0.1 M x V1]/[0.05 M] = 2V1. This means that we need twice the volume of HCl than the volume of NaOH.
Next, we need to measure out the appropriate amount of each solution. For this experiment, we will use 10mL of HCl and 5mL of NaOH. We will then add the HCl to a beaker containing the NaOH and stir until the solution turns a light pink. This indicates that the acid and base have been neutralized and the titration is complete.
I hope this example helps you better understand HCl and how to use it in a titration experiment. If you have any other questions, please feel free to let me know.
Properties of Hydrogen Chloride
Hydrogen Chloride (HCl) is a colourless, sharp-smelling gas composed of hydrogen and chlorine atoms. It is a highly corrosive, strong acid that readily dissolves in water, forming hydrochloric acid. It is commonly used in the production of plastics, textiles, dyes, pharmaceuticals, and other industrial processes. Some of its properties include:
Boiling point: -85.05 °C
Melting point: -114.2 °C
Density: 1.56 g/L
Solubility: Highly soluble in water
pH: 0.5 (Strongly acidic)
Viscosity: 0.0027 Pa·s at 25 °C
Molecular weight: 36.461 g/mol
Chemical Properties of Hydrogen Chloride
Hydrogen chloride, or HCl, is a colorless, corrosive gas with a pungent odor. It is a compound of the elements hydrogen and chlorine. When exposed to humid air, HCl forms an acidic mist. It is highly soluble in water, forming hydrochloric acid, which is an important commercial chemical. HCl is used as a source of chlorine in the production of numerous industrial chemicals, including PVC and other plastics. In addition, it is used in the production of pharmaceuticals, dyes, and food additives. HCl is highly corrosive and can cause burns to skin and eyes. Inhalation of the gas can cause breathing difficulties and irritation to the throat and lungs.
Frequently Asked Questions – FAQs
Hydrochloric acid is prepared by adding aqueous potassium chloride solution to concentrated sulfuric acid in a ratio of 1:1.
The laboratory preparation of hydrochloric acid to avoid back suction would involve using a flask with a rubber stopper, on a mechanical stirrer, and on a heating mantle.
HCl is prepared in a chemical reaction that uses a catalyst.A catalyst is a chemical that makes the reaction happen faster.The catalyst is typically a metal that reacts with the hydrogen to form hydrogen gas.The hydrogen gas then reacts with the hydrogen chloride to form hydrochloric acid.
related content
🌟 Conditions for the formation of an electrovalent (or ionic) bond class 10 ICSE
🌟 Understanding Hydrogen Chloride Formula: A Quick Guide For Class 10 ICSE Students
